I. The Basics Question 1: As an employee of the JHM covered entity, how does the HIPAA Privacy Rule affect my research? Answer: Under the HIPAA Privacy Rule you must meet certain requirements before using or disclosing individually identifiable health information for research.
Methods for De-identification of PHI | HHS.gov
IRB, Investigators should include the “HIPAA AUTHORIZATION TO USE HEALTH INFORMATION FOR RESEARCH” with the informed consent document and other application documents. 3. Authorization May Still Be Needed . For waived research that does not require an Informed Consent (e.g., retrospective records research), a HIPAA authorization may still be

Source Image: link.springer.com
Download Image
transmits health information in electronic form in connection with a transaction for which HHS has adopted a standard. Protected Health Information – PHI is individually identifiable health information transmitted by electronic media, maintained in electronic media, or transmitted or maintained in Health Information – Research

Source Image: revivalresearch.org
Download Image
What Are the top 5 Components of the HIPAA Privacy Rule? Under the HIPAA Privacy Rule, covered entities may use or disclose protected health information from existing databases or repositories for research purposes either with individual authorization as required at 45 CFR 164.508, or with a waiver of individual authorization as permitted at 45 CFR 164.512 (i). Read the full answer

Source Image: slideshare.net
Download Image
Hipaa’S Protections For Health Information Used For Research Purposes
Under the HIPAA Privacy Rule, covered entities may use or disclose protected health information from existing databases or repositories for research purposes either with individual authorization as required at 45 CFR 164.508, or with a waiver of individual authorization as permitted at 45 CFR 164.512 (i). Read the full answer Center for Medicare & Medicaid Services HIPAA Information (Covered Entity Decision Tool) Final HIPAA Enforcement Rule ( PDF / TXT ) OCR Issues the HITECH Breach Notification Interim Final Regulation August 24, 2009
Electronic Medical Records and Meaningful Use | PPT
The following is a privacy policy language profile proposal for HIPAA-Compliant e-Health Applications, published by Elsevier B.V. Included in the proposition is the aim of usage allowing the e-health providers to specify HIPAA-compliant privacy policies and the ability for patents to be able to expr Medical record – Wikipedia

Source Image: en.wikipedia.org
Download Image
DataGuidance The following is a privacy policy language profile proposal for HIPAA-Compliant e-Health Applications, published by Elsevier B.V. Included in the proposition is the aim of usage allowing the e-health providers to specify HIPAA-compliant privacy policies and the ability for patents to be able to expr
Source Image: dataguidance.com
Download Image
Methods for De-identification of PHI | HHS.gov I. The Basics Question 1: As an employee of the JHM covered entity, how does the HIPAA Privacy Rule affect my research? Answer: Under the HIPAA Privacy Rule you must meet certain requirements before using or disclosing individually identifiable health information for research.
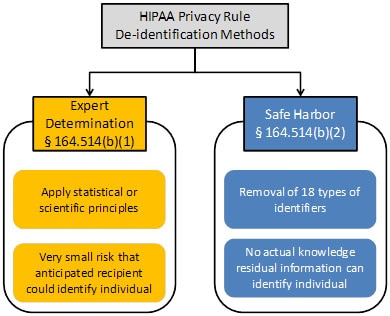
Source Image: hhs.gov
Download Image
What Are the top 5 Components of the HIPAA Privacy Rule? transmits health information in electronic form in connection with a transaction for which HHS has adopted a standard. Protected Health Information – PHI is individually identifiable health information transmitted by electronic media, maintained in electronic media, or transmitted or maintained in Health Information – Research

Source Image: blog.rsisecurity.com
Download Image
HIPAA Compliance, Data Breaches, and Cybersecurity News and Insights – HealthITSecurity HIPAA’s privacy provisions are limited to use and disclosure of Protected Health Information, or PHI. PHI is defined as individually identifiable health information that is created or received by a HIPAA covered entity Health information includes any information, whether oral or recorded in any form, that relates to the

Source Image: healthitsecurity.com
Download Image
HIPAA’s Protections For Health Information Used For Research Purposes Under the HIPAA Privacy Rule, covered entities may use or disclose protected health information from existing databases or repositories for research purposes either with individual authorization as required at 45 CFR 164.508, or with a waiver of individual authorization as permitted at 45 CFR 164.512 (i). Read the full answer

Source Image: revivalresearch.org
Download Image
What is HIPAA? Importance of HIPAA Compliance Center for Medicare & Medicaid Services HIPAA Information (Covered Entity Decision Tool) Final HIPAA Enforcement Rule ( PDF / TXT ) OCR Issues the HITECH Breach Notification Interim Final Regulation August 24, 2009
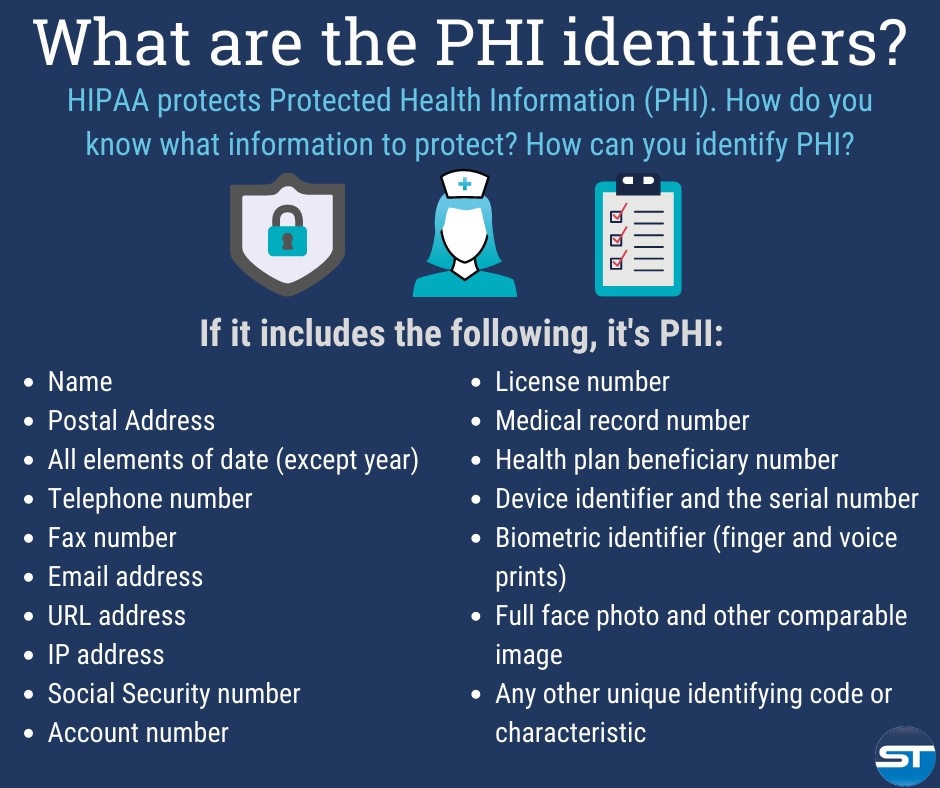
Source Image: smarttraining.com
Download Image
DataGuidance
What is HIPAA? Importance of HIPAA Compliance IRB, Investigators should include the “HIPAA AUTHORIZATION TO USE HEALTH INFORMATION FOR RESEARCH” with the informed consent document and other application documents. 3. Authorization May Still Be Needed . For waived research that does not require an Informed Consent (e.g., retrospective records research), a HIPAA authorization may still be
What Are the top 5 Components of the HIPAA Privacy Rule? HIPAA’s Protections For Health Information Used For Research Purposes HIPAA’s privacy provisions are limited to use and disclosure of Protected Health Information, or PHI. PHI is defined as individually identifiable health information that is created or received by a HIPAA covered entity Health information includes any information, whether oral or recorded in any form, that relates to the